Abstract
Introduction Waldenstrom Macroglobulinemia (WM) is a rare non-Hodgkin lymphoma that is preceded by an IgM monoclonal gammopathy of undetermined significance (IgM-MGUS), a premalignant, clinically asymptomatic disorder. The factors underlying this malignant transformation remain poorly understood, but the stepwise accumulation of genomic abnormalities has been identified as a potential mechanism. Non-coding aspects of the genome are increasingly recognized as drivers of disease pathogenesis but remain under-explored in this disease spectrum. Previously, we have shown that several oncogenes are in close genomic proximity to overexpressed long non-coding RNAs in WM compared to IgM-MGUS (Paludo et al. 2019). In this study, we aimed to identify the microRNAs (miR) and genomic pathways which may facilitate the IgM-MGUS to WM malignant progression.
Methodology Sorted CD19+ and CD138+ B cells from prospectively collected bone marrow samples of patients with IgM-MGUS (n=7) and WM (n=25) underwent total RNA extraction and sequencing. Profiling of miRNAs was conducted, and selection of targets of interest was performed through identifying differentially expressed miRNA between IgM-MGUS and WM with a log2 fold change (FC)>0.5 or <-0.5 and false discovery rate (FDR) <0.05. Next, mRNA sequencing was conducted on the same patient samples, and differential expression was performed the same way as the miRNA data to identify mRNA candidates. Differentially expressed miRNA/mRNA pairs between WM and IgM-MGUS with correlated biological expression, i.e. either upregulated (up) miRNA experimentally predicted to regulate downregulated (down) mRNA or vice versa, were selected. The miRNA/mRNA pairs identified here were evaluated using Ingenuity Pathway Analysis (IPA) to discover dysregulated genomic pathways between IgM-MGUS and WM. Only pathways with an absolute activation Z-score of ≥2 were selected, and dysregulated miRNA and associated genes involved in altered pathways were identified.
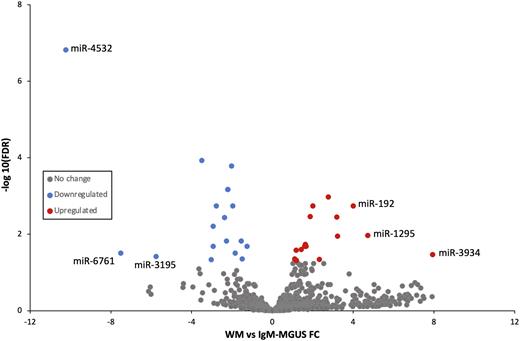
Results There were 34 miRNAs (16 up/18 down) found to be significantly differentially expressed between IgM-MGUS and WM (Figure). Among these, miR-4532, miR-192, miR-3195, miR-6761, miR-1295, and miR-3934 were found to have the highest FC between these two conditions. After correlation of miRNA/mRNA pairs, the most differentially regulated pathways with recognized oncological relations were identified, among which, the ID-1, IL-8 and STAT3 pathways were found to be downregulated in WM compared to IgM-MGUS, while the PTEN pathway was found to be upregulated. More broadly, the G protein-coupled receptor (GPCR), and the tumor microenvironment (TM) pathways were both found to be downregulated in WM compared to IgM-MGUS. Some of the main miRNAs driving multiple pathways with key associated genes included: miR-20 (GPCR, TM, IL8, ID1) through regulation of cyclin D1 (CCND1), matrix metalloproteinase-2 (MMP2), and leukemia inhibitory factor (LIF), miR-1260 (ID1, GPCR, STAT3, TM) through regulation of B-cell lymphoma 2 (BCL-2), chemokine receptor 6 (CCR6), and ETS proto-oncogene 1 (ETS1), and miR-1295 (IL8, TM, GPCR) through regulation of vascular cell adhesion molecule 1 (VCAM1), matrix metallopeptidase 19 (MMP19), and hydroxycarboxylic acid receptor 2 (HCAR2).
Conclusion Our study is one of the first to identify the altered non-coded transcriptomics in WM and MGUS. We found several differentially expressed miRNAs with a known oncological role, such as miR-4532, miR-20 and miR-1260, highlighting the role of miRNAs as potentially important epigenetic regulators in these two diseases. The genomic pathways found to be potentially regulated by miRNA may underlie the transition between the premalignant to malignant disease of IgM-MGUS to WM.
Disclosures
Kapoor:Sanofi: Honoraria, Research Funding; X4 Pharmaceuticals: Honoraria; Regeneron: Research Funding; Amgen: Research Funding; Ichnos: Research Funding; Loxo: Research Funding; Karyopharma: Research Funding; BMS: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Research Funding; Casma: Honoraria; Pharmacyclics: Honoraria; Imedex: Honoraria; GSK: Honoraria; Cellectar: Honoraria; Oncopeptides: Honoraria. Novak:Bristol Myers Squibb: Research Funding. Ansell:SeaGen: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal